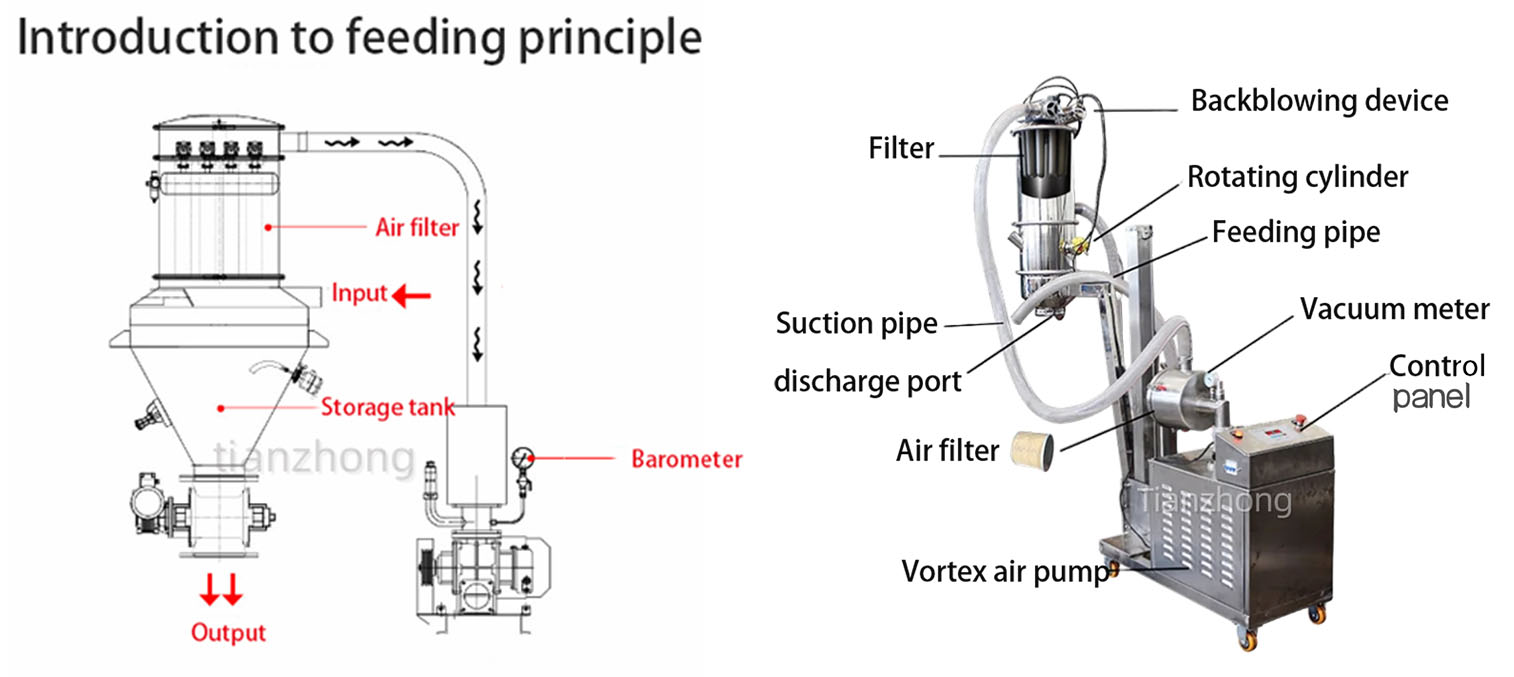
MORE ABOUT US
About Tianzhong Machinery
Henan Tianzhong Vibration Equipment Co., Ltd. integrates “research and development, production, sales and service”, with an annual production capacity of more than 5,000 units, and its products cover the three major fields of vibrating screening, conveying and lifting, and dust removal, including rotary vibrating screen, vacuum feeder, dust-free feeding station, ton bag feeding station, and customized various kinds of dust-free powder supply system, which are committed to dozens of series of dust-free powder screening and mixing one-stop solutions. The products are widely used in medicine, chemical industry, abrasives, ceramics, mining, food, metallurgy and other industries, and for a long time for the domestic more than 150 counterparts to provide OEM services, and many large-scale enterprises to establish long-term supporting cooperation. Relying on standardized CAD design and strict quality control system, our products are known for high precision and stability, and are exported to the United States, France, Russia, Australia and Southeast Asia and other countries and regions.